

Microscope image of cerebral organoid derived from human cells showing neuronal progenitors (green) and neurons (red) (image: Fábio Papes/UNICAMP)
Researchers at the State University of Campinas and the University of California San Diego have discovered the mechanism that causes this rare but severe autism spectrum disorder. They reversed progression of the syndrome in laboratory models, opening up new possibilities for treatment using drugs and gene therapy.
Researchers at the State University of Campinas and the University of California San Diego have discovered the mechanism that causes this rare but severe autism spectrum disorder. They reversed progression of the syndrome in laboratory models, opening up new possibilities for treatment using drugs and gene therapy.
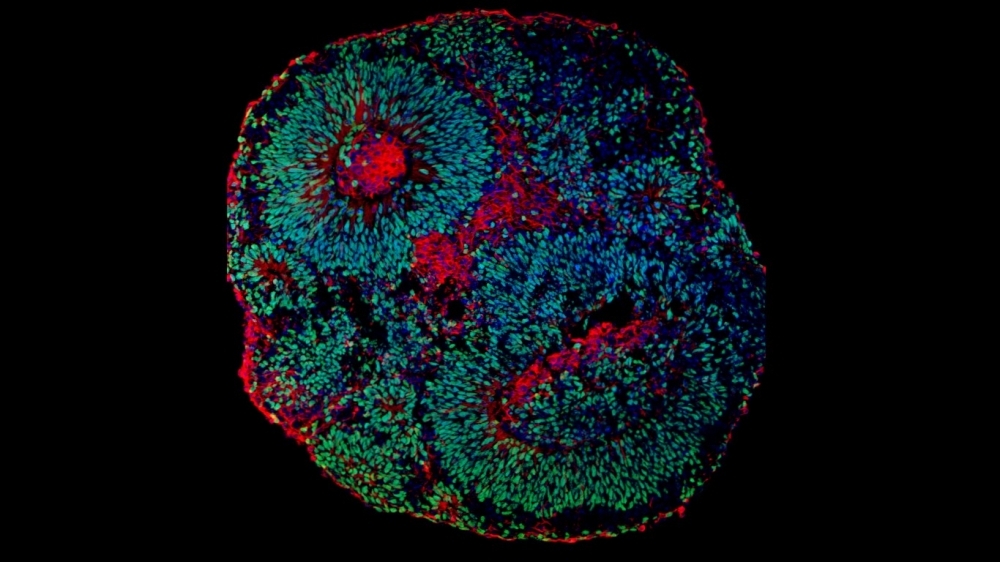
Microscope image of cerebral organoid derived from human cells showing neuronal progenitors (green) and neurons (red) (image: Fábio Papes/UNICAMP)
By André Julião | Agência FAPESP – A group led by Brazilian scientists at the State University of Campinas (UNICAMP) in São Paulo state and the University of California San Diego (UCSD) in the United States has discovered the mechanism that causes Pitt-Hopkins syndrome (PTHS), a neuropsychiatric disorder with autism spectrum disorder characteristics. The researchers also succeeded in reversing progression of the syndrome in laboratory models, opening up new possibilities for treatment.
The study was supported by FAPESP. An article describing it is published in Nature Communications.
“For most cases of autism spectrum disorder, the mutant gene that caused the condition is unknown. The same is true of most neuropsychiatric diseases, such as schizophrenia, depression or bipolar disorder. PTHS is known to be caused by a mutation in the gene TCF4, but until now nothing was known about its molecular mechanisms, or what’s different about the nervous system cells of patients with the mutation,” said Fabio Papes, a professor in the Institute of Biology (IB-UNICAMP) and first author of the article.
The group led by Papes and Alysson Muotri, a professor at UCSD, went beyond discovering the mechanism underlying the condition by testing ways of interfering in its progression and succeeded in reversing the effects of the mutation. The positive results of their experiments pave the way for the development of medications and potentially for genetic therapy.
PTHS is characterized by intellectual disability, a marked delay in motor development, lack of functional speech and disordered breathing, among other problems. It was described in 1978, but the gene that causes it was not discovered until 2007. The mutation in TCF4 is believed to occur once in 35,000 births.
Mini-brains
Studying PTHS in mice or other laboratory animals is pointless because it does not develop in them in the same way as in humans, so the researchers used cerebral organoids or “mini-brains” – clusters of human cells grown in the laboratory until they resemble miniature developing brains, albeit without vascularization and with fewer cell types.
“Cerebral organoids are more representative models than any other for the study of central nervous system dysfunctions,” Papes said. “In this case, the cells are donated by the patient, and the organoid is three-dimensional, functioning much more similarly to the human brain than cells cultured in a Petri dish and hence in only two dimensions.”
The organoids derived from skin biopsies obtained from PTHS patients recruited at UNICAMP and UCSD. Skin samples from their parents were used as controls. The cells were cultured to obtain fibroblasts, which were transformed into pluripotent stem cells capable of generating many types of human cell. In this case, they gave rise to neuronal progenitor cells, forerunners of neurons and key to the central nervous system and cerebral organoids.
While the parental cells formed organoids that developed normally, the cells from PTHS patients grew less owing to a lower replication rate caused by the mutation, which impaired neurogenesis. Furthermore, neurons in the organoids with the mutant TCF4 were fewer in number and had less electrical activity than neurons in the control organoids. Communication among these cells is known to require electrical pulses, without which they cannot perform their functions. This finding may therefore explain many clinical features of PTHS patients.
The results were similar to those obtained in brain tissue from a PTHS patient who died for other reasons, reinforcing the conclusions drawn from the experiments with the organoids. As far as the researchers are aware, their study was the first to investigate the brain of a PTHS patient.
“Access to the post-mortem brain was essential to validate some of the results obtained with the cerebral organoids,” Muotri said. “The fact that we found similar features in the lab-grown organoid and the brain shows how relevant this technology is.”
Gene therapy
Having observed the alterations caused by the mutation in the gene TCF4, the researchers sought ways of correcting it. This entailed performing what is known as proof of concept for potential treatment.
Three interventions were tested. One used CRISPR-Cas9, the gene editing technique whose creators won the 2020 Nobel Prize in Chemistry.
A recent version of the CRISPR-Cas9 technique was deployed to make a functional copy of the gene present in the dysfunctional cell express much more protein and offset the copy affected by the mutation that causes PTHS.
In the second intervention using a different technique, the scientists inserted an extra copy of the gene, which performed its functions normally to compensate for the mutant copy.
“Our genome has two copies of every gene. What causes PTHS is the fact that one of the copies of TCF4 doesn’t work. Inserting a third copy, or making the only functional copy express more protein to offset the defective one can solve the problem,” Papes said.
The organoids altered by the interventions began growing normally, and proliferation of their progenitor cells increased. In the actual brain, these give rise to different types of cells, including neurons.
“PTHS is a rare disease, but others also involve mutations in the same gene, so our discovery can be used in future to treat disorders like schizophrenia, for example,” Papes said.
A third intervention consisted of the administration of CHIR99021, a drug used in studies involving tumor cells that activates a cell signaling pathway known as Wnt, widely studied for cancer research. The authors discovered that Wnt is also altered by mutations in the gene TCF4.
In dysfunctional cells and organoids treated with the drug, several molecular indicators improved and organoid size increased. The results open up the possibility of developing similar medications to treat the dysfunction since CHIR99021 cannot yet be used in humans.
“This pathway treated with the drug is merely one of those altered by the gene mutation. The advantage of gene therapy over pharmacological treatment is that it would solve the problem at its source. However, the search for new drugs is also promising,” Papes said.
The researchers will now advance to pre-clinical and clinical trials, partnering with a company which is specialized in gene therapy and is licensing the technology used in the experiments for future testing on human patients.
The study was supported by FAPESP via a master’s scholarship awarded to José Ricardo Teixeira Júnior and a PhD scholarship awarded to Antônio Camargo, both affiliated with IB-UNICAMP.
Funding for the research was also provided by the National Scientific and Technological Development Council (CNPq) in Brazil, the National Institutes of Health (NIH) in the United States, and the Pitt-Hopkins Research Foundation.
The article “Transcription Factor 4 loss-of-function is associated with deficits in progenitor proliferation and cortical neuron content” is at: www.nature.com/articles/s41467-022-29942-w.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





