

CAR T-cell therapy offers hope for people with types of cancer that are hard to treat (illustration: CTC-USP)
Opened in 2022 with FAPESP’s support, Nutera is Latin America’s first cellular product manufacturing plant. It is run by researchers from Butantan Institute and the University of São Paulo’s Ribeirão Preto Blood Center, and could become a supplier to the SUS, Brazil’s public healthcare network.
Opened in 2022 with FAPESP’s support, Nutera is Latin America’s first cellular product manufacturing plant. It is run by researchers from Butantan Institute and the University of São Paulo’s Ribeirão Preto Blood Center, and could become a supplier to the SUS, Brazil’s public healthcare network.
CAR T-cell therapy offers hope for people with types of cancer that are hard to treat (illustration: CTC-USP)
By Maria Fernanda Ziegler | Agência FAPESP – Approved on September 27 by ANVISA, Brazil’s national health surveillance agency, a clinical trial of chimeric antigen receptor (CAR) T-cell therapy for blood cancer is set to begin in the coming months, with 81 volunteers who will now be selected. According to the trial’s designers, it will be the first step to transform the Nutera Advanced Therapy Center, Latin America’s first cellular product manufacturing plant, opened in 2022 with FAPESP’s support, into a supplier of products for advanced cell therapies, such as CAR T-cells, to the SUS (Sistema Único de Saúde), Brazil’s public healthcare network.
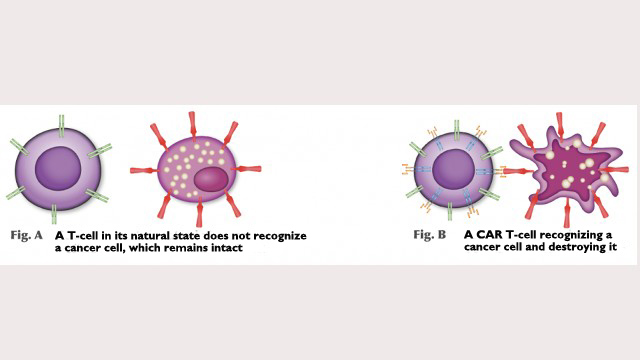
CAR T-cell therapy offers hope for people with types of cancer that are hard to treat. It was first trialed in Latin America in 2019 by researchers at the Center for Cell-Based Therapy (CTC), a Research, Innovation and Dissemination Center (RIDC) established by FAPESP at the University of São Paulo (USP) in Ribeirão Preto. To date, the experimental treatment has been administered to 17 patients at CTC, with full remission within about a month in most cases (read more at: agencia.fapesp.br/31675).
The 81-patient Phase 1 and 2 clinical trial will confirm the safety and efficacy of the treatment for patients with B-cell acute lymphoid leukemia (ALL) and B-cell non-Hodgkin lymphoma (NHL). Approval is pending from the National Commission of Ethics in Research (CONEP). The volunteer profile will be established once its approval has been obtained.
The trial will take place free of charge at five hospitals in São Paulo state, including the general and teaching hospitals run by the university’s medical school in Ribeirão Preto (Hospital das Clínicas/FMRP-USP) and the state capital (HC/FM-USP).
“Approval of the trial is important as a step toward producing cellular products [cells altered in the laboratory] for the treatment of different kinds of cancer and other diseases. It’s the first time such complex products are to be distributed entirely in Latin America and a huge advance for the area of biotechnology,” said Dimas Covas, head of the Ribeirão Preto Blood Center and principal investigator for the research project.
ANVISA has also authorized production of cellular products for the trial. The Ribeirão Preto Blood Center, USP and Butantan Institute are partnering in Nutera, which has received funding from FAPESP’s Science for Development Centers Program (read more at: agencia.fapesp.br/39048).
Once the results of the trial are in, applications can be made to ANVISA for permission to market cellular products. “The manufacturing plant is a platform for manipulating genetically modified cells like CAR T-cells, as well as viral vectors for gene therapy and cells derived from pluripotent stem cells. The facility is on public land in a hospital that almost exclusively admits patients on the SUS. We began with CART19, but we have a research pipeline and will soon be producing second- and third-generation CAR T-cell products, for example. The clinical trial is just the start of a highly successful project,” Covas said.
The plant currently has the capacity to produce cells for 13 patients every 34 days. “It’s not sufficient to meet all the demand from the SUS, but we expect to be able to cater for 150 patients within a year and continue scaling up thereafter,” said Diego Clé, medical director of the Ribeirão Preto Blood Center and one of the researchers involved.
Cell by cell
In the clinical trial, T-lymphocytes (defense cells) will be collected from the volunteers and sent to the cellular product plant, where the researchers will use bioengineering techniques to insert a viral vector gene sequence into the T-cells’ DNA.
The T-cells’ new DNA will express a receptor that was not there before and can recognize tumors. “The lymphocytes express CAR, the receptor, which recognizes a specific protein in tumor cells. In the case of our study, it recognizes CD19, a protein present in lymphoid leukemia and non-Hodgkin lymphoma cells,” Clé explained.
The genetically modified lymphocytes will be deep-frozen and sent back to the hospitals where the trial is being held. “The patients will receive their new lymphocytes as if they were having a blood transfusion. The CAR T-cells recognize the tumor and release a number of inflammatory mediators to attack it,” he said.
For more information (in Portuguese), visit: www.hemocentro.fmrp.usp.br/terapia/.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





