

Nonalcoholic fatty liver disease is commonly associated with polycystic ovary syndrome, which affects up to 15% of women of reproductive age. Study could lead to the development of less invasive diagnostic methods (image: Scientific Reports)
Nonalcoholic fatty liver disease is commonly associated with polycystic ovary syndrome, which affects up to 15% of women of reproductive age. Study could lead to the development of less invasive diagnostic methods.
Nonalcoholic fatty liver disease is commonly associated with polycystic ovary syndrome, which affects up to 15% of women of reproductive age. Study could lead to the development of less invasive diagnostic methods.
Nonalcoholic fatty liver disease is commonly associated with polycystic ovary syndrome, which affects up to 15% of women of reproductive age. Study could lead to the development of less invasive diagnostic methods (image: Scientific Reports)
By Maria Fernanda Ziegler | Agência FAPESP – Researchers at the University of São Paulo Medical School (FM-USP) in Brazil have identified a biological marker of nonalcoholic fatty liver disease (NAFLD), a condition in which excess fat builds up in the liver cells of people who drink little or no alcohol.
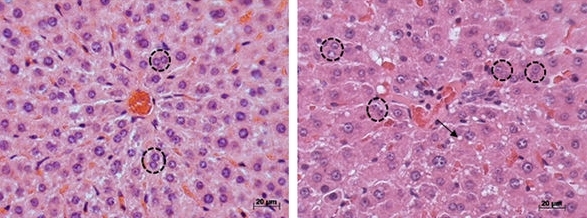
The biomarker was discovered in rats, and its occurrence in humans must now be confirmed. NAFLD is currently diagnosed on the basis of laboratory tests and an ultrasound scan, with a liver biopsy for confirmation if necessary. This invasive procedure may pose a risk to the patient’s health.
NAFLD is commonly associated with polycystic ovary syndrome (PCOS), a hormone imbalance leading to anovulation (lack of ovulation) or irregular ovulation, which is present in as many as 15% of women of reproductive age depending on the criteria used. Although NAFLD is believed to be a consequence of insulin resistance in patients with PCOS, the mechanisms involved in the development of the liver pathology are unknown.
In an article published in the journal Scientific Reports, the researchers report their analysis of metabolites produced by the breakdown of glucose, amino acids and lipids using mass spectrometry for targeted metabolomic profiling based on a model of PCOS in rats.
The FM-USP research group led by Professor Gustavo Maciel demonstrated that there were alterations in the livers of rats with PCOS that were very similar to NAFLD in humans. Moreover, they found that increased levels of branched-chain amino acids – leucine, isoleucine and valine, which are essential amino acids – can be used as a marker for NAFLD.
The study, conducted at the FM-USP Structural & Molecular Gynecology Laboratory (LIM-58), resulted from a research project supported by FAPESP and was part of the master’s research of physician Alvaro Anzai, who collaborated with researchers at the Federal University of São Paulo (UNIFESP) in Brazil and the University of Michigan in the United States.
The researchers divided 30 two-day-old female rats into three groups, each receiving a different treatment. To induce PCOS, the first group received 1.25 mg of the hormone testosterone; the second group was given 0.5 mg of estradiol. The control group received only placebo.
The animal model with estradiol induction produced a milder phenotype of the syndrome. Although ovulation did not occur, the group displayed neither metabolic impairment nor high testosterone levels (common in cases of PCOS). The group with testosterone-induced PCOS displayed the main alterations typical of the most severe form of the syndrome: chronic anovulation, insulin resistance, high testosterone levels and fatty liver.
The metabolites produced by the various cellular processes in the animals’ livers were quantified, and specific patterns were identified for each group. On this basis, the researchers developed hypotheses regarding the mediators of PCOS and the pathways involved in its evolution.
“In addition to investigating alterations in the histological part of the study, we also analyzed metabolite dosage using bioinformatics. The conclusion was that there appeared to be a deficit in the metabolism of branched-chain amino acids, high levels of which were found in the rats’ liver tissue. We now plan to see whether this biomarker can also be identified in blood samples,” said Rodrigo Marcondes, a coauthor of the article.
The expectation is that the metabolic signature will be reproduced not only in histological testing but also in the blood. According to Maciel, the group’s preliminary studies performed in collaboration with Ismael Dale, a professor at UNIFESP’s Medical School (Escola Paulista de Medicina, EPM), point to a rise in the blood levels of branched-chain amino acids in patients with insulin resistance.
New research lines
The discovery of a possible biomarker for NAFLD is part of only one among several research lines pursued by the FM-USP group. The next step will be to analyze alterations in branched-chain amino acids in humans as part of a new research project involving patients treated at FM-USP’s general and teaching hospital (Hospital das Clínicas).
“The initial aim was to use metabolomics to identify a marker of hepatic alteration in these animal models,” Maciel said. “This study gave rise to new research lines. The idea now is to validate the same markers in women with PCOS.”
According to Maciel, confirmation of the hypothesis that the biomarker behaves similarly in humans will significantly advance diagnosis and treatment of the disease. “We’re looking for a noninvasive method of diagnosing NAFLD for use in clinical practice,” he said.
The researchers are also studying the effects of physical exercise on general metabolic aspects of PCOS and investigating whether it reduces the levels of branched-chain amino acids.
“Physical exercise is normally indicated as the first line of treatment for PCOS, but the action mechanisms of the syndrome are poorly understood, and this study may provide a better understanding of its physiopathology and strategies for treatment,” Marcondes said.
Another line of research, also supported by FAPESP, aims to estimate the risk of metabolic problems in patients with PCOS.
“The idea is to use biological markers to predict which patients with PCOS could develop metabolic problems, such as an increased risk of diabetes and fatty liver, for example,” Maciel said.
The Scientific Reports article “Impaired branched-chain amino acid metabolism may underlie the nonalcoholic fatty liver disease-like pathology of neonatal testosterone-treated female rats” (doi: 10.1038/s41598-017-13451-8) by Álvaro Anzai, Rodrigo R. Marcondes, Thiago H. Gonçalves, Kátia C. Carvalho, Manuel J. Simões, Natália Garcia, José M. Soares Jr, Vasantha Padmanabhan, Edmund C. Baracat, Ismael D. C. G. da Silva and Gustavo A. R. Maciel can be read at nature.com/articles/s41598-017-13451-8.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





