

The proposition is to use nanoparticles that can be guided by applying an external magnetic field to attack solid tumors resistant to conventional treatment (image: researchers’ archive)
The proposition is to use nanoparticles that can be guided by applying an external magnetic field to attack solid tumors resistant to conventional treatment.
The proposition is to use nanoparticles that can be guided by applying an external magnetic field to attack solid tumors resistant to conventional treatment.
The proposition is to use nanoparticles that can be guided by applying an external magnetic field to attack solid tumors resistant to conventional treatment (image: researchers’ archive)
By Janaína Simões | Agência FAPESP – Researchers at the Federal University of the ABC (UFABC) in the state of São Paulo, Brazil, have developed a nanotechnology platform for targeted treatment of tumors without affecting healthy tissue and with less risk of side-effects. To do so, they used a property called superparamagnetism to ensure that the nanoparticles deliver the compound of interest only to cancer cells.
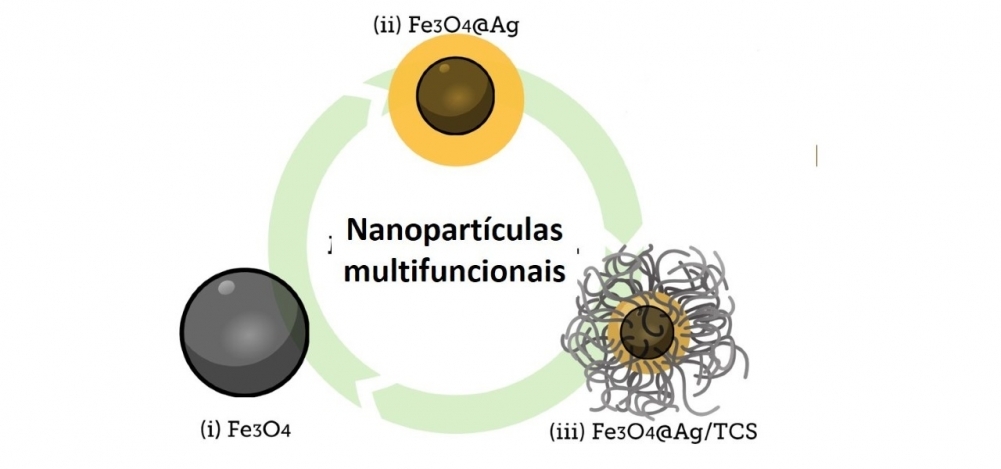
“We’ve created a novel material, an original. It consists of a magnetic core of magnetite [Fe3O4] to which we add silver [Ag] nanoparticles coated with a polymer containing a nitric oxide [NO] donor. It has low toxicity and good biocompatibility. We’ve applied to National Institute of Industrial Property [INPI, the Brazilian patent office] for a patent,” said Amedea Barozzi Seabra, a professor at UFABC and one of the scientists involved in the project. She conducted the research in collaboration with colleagues at the State University of Londrina (UEL) in Paraná state, Brazil, and the University of Lorraine (UL) in France.
According to Seabra, a magnetic compound can be guided to the target site by applying an external magnetic field. In the case of superparamagnetism, the nanoparticles align in the same direction at room temperature when the magnetic field is applied. When it is removed, they become randomly oriented and immediately return to the initial state without residual magnetization. “It’s like using a light switch. You press the switch and turn on the light. You press it again and the light goes out. All this happens at room temperature,” she said. In other words, superparamagnetic substances do not retain magnetization once the external field has been removed, and have no magnetic memory.
Joana Claudio Pieretti, first author of an article on the study published in the Journal of Materials Science: Materials in Medicine, was supervised by Seabra for her master’s research resulting in the nanotechnology platform, with funding from FAPESP (18/08194-2; 19/07766-5; and 18/02832-7). Both are affiliated with UFABC’s Center for Natural and Human Sciences (CCNH).
Interest in versatile multifunctional materials is currently high among scientists, Seabra explained, citing the example of moisturizing agents in cosmetics that also improve skin firmness and smooth expression wrinkles. “We set out to develop a single material and add functionality to it. We added elements to the nanoparticle, each with a function, to arrive at a compound capable of treating various kinds of tumor,” she said.
Magnetite was chosen because it is superparamagnetic. Silver nanoparticles were added to this material. They were obtained from green tea, which is rich in antioxidants such as polyphenols and caffeine. “These molecules act as powerful reducing agents, reducing the silver ions to form silver nanoparticles, while also helping to stabilize them,” Pieretti said.
Silver nanoparticles have long been used to combat bacterial infections and tumors. Both types of action are boosted by coating them with the molecules extracted from green tea. “This was not yet sufficient, however, so we added modified chitosan, a biodegradable polymer derived from crustaceans, as an extra coating that releases the nitric oxide while also assisting the anti-bacterial and anti-tumor action,” she explained.
Tests in cells
In vitro testing was carried out using prostate and bone cancer cell lines. “Previous studies had focused on developing nanoparticles to treat specific types of tumor, but we wanted a more versatile material to treat different types of solid tumor including breast and prostate tumors because in these cases it’s possible to guide the nanoparticles to the site of interest. The goal is to use the nanocompound to treat solid tumors resistant to chemotherapy and radiation therapy, which are the hardest to treat,” Seabra said.
“Our nanoparticle is directional as it uses a superparamagnetic compound, so it wouldn’t be suitable for treatment of leukemia, for example, which is in the bloodstream and not a specific organ,” Pieretti said.
Blood compatibility
The researchers characterized the nanocompound physically, chemically and morphologically, analyzed its therapeutic efficacy, and conducted biological trials to confirm its anti-tumor action and measure nitric oxide release. They also analyzed its hemocompatibility (interaction with blood cells). “We can’t risk placing something in the bloodstream to combat a tumor if it may throw a clot, for example,” Seabra said.
This part of the research was conducted at UL in Nancy, France, where Pieretti worked as an intern with FAPESP’s support, and was recently reported in the International Journal of Molecular Science. According to the authors, “We observed that the more stable nanoparticles (coated with modified chitosan) present a lower affinity for albumin in comparison to pure magnetite and magnetite/silver hybrid nanoparticles. Surface properties were key players at the nanoparticle–biological interface.”
Hyperthermia
The nanotechnology platform also has the potential to be used in the treatment of cancer by hyperthermia, in which temperature is raised in the tumor using superparamagnetic nanoparticles and a magnetic field, in order to disrupt proteins and kill tumor cells. Nitric oxide is also an anti-tumor agent.
Superparamagnetic nanoparticles can be rapidly aligned and disaligned, generating energy to raise local temperature. “This is a possible application for the platform, but has yet to be evaluated,” Seabra said.
In vivo trials in collaboration with other research groups are required in this case. Pieretti is currently doing PhD research that involves further work on nanoparticles and nitric oxide to combat cancer. She plans more research abroad to conduct in vitro and possibly in vivo experiments focusing on liver cancer.
The article “Multifunctional hybrid nanoplatform based on Fe3O4@Ag NPs for nitric oxide delivery: development, characterization, therapeutic efficacy, and hemocompatibility” is at: link.springer.com/article/10.1007/s10856-021-06494-x.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





