

Immunofluorescence microscope image of Vero E6 cells infected with SARS-CoV-2. Detail magnified on the right clearly shows vesicles (credit: Edison Durigon/ICB-USP)
Collaboration between business and academia in the state of São Paulo proves the virucidal action of iron phthalocyanine and develops a mouthwash containing the compound. In a clinical trial involving patients in the initial stage of infection, the product reduces symptoms and hospital stay.
Collaboration between business and academia in the state of São Paulo proves the virucidal action of iron phthalocyanine and develops a mouthwash containing the compound. In a clinical trial involving patients in the initial stage of infection, the product reduces symptoms and hospital stay.
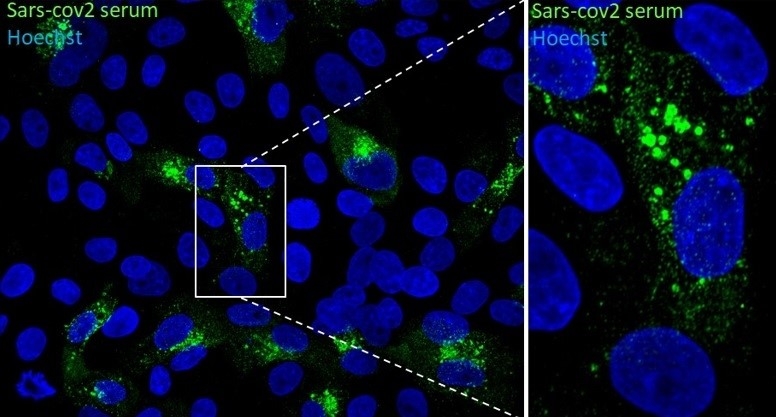
Immunofluorescence microscope image of Vero E6 cells infected with SARS-CoV-2. Detail magnified on the right clearly shows vesicles (credit: Edison Durigon/ICB-USP)
By Elton Alisson | Agência FAPESP – In Brazil, researchers at the University of São Paulo’s Institute of Chemistry (IQ-USP) discovered that a molecule derived from the textile dye phthalocyanine inactivates SARS-CoV-2. Working with scientists at a chemicals manufacturer in the state of São Paulo, they have developed a process to produce it to scale for inclusion in a mouthwash.
In laboratory tests performed at the university’s Biomedical Sciences Institute (ICB-USP) and reported in the journal Scientific Reports, the compound reduced viral load in cell cultures by 99.96% without cytotoxic effects (cell damage or death).
A clinical trial conducted by researchers at its Bauru Dental School (FOB-USP) showed that intensive use of a mouthwash containing the antiviral compound by patients at a public hospital in the city reduced the severity of symptoms in the initial stage of infection and shortened hospital stay.
The trial and laboratory tests were supported by FAPESP.
“The molecule binds to the oxygen present in air. When this reaction occurs, the oxygen becomes more active and damages the virus by oxidation,” Koiti Araki, a professor at IQ-USP and principal investigator for the project, told Agência FAPESP.
Phthalocyanines are the second most important class of colorant, widely used in textile dyes, automotive paints and printing inks. Iron phthalocyanine has anion groups (negatively charged ion groups) that activate the iron ion at the center of the molecule, which binds to the oxygen in the air and makes it reactive. In this form, oxygen behaves like ozone or hydrogen peroxide, causing oxidative damage to viruses, bacteria and other microorganisms.
In recent years, Araki and his group have partnered with researchers at Golden Technology, a company headquartered in São José dos Campos (São Paulo state), to develop a process to produce the molecule to scale. “It’s hard to produce, and yields have typically been very low, but we’ve succeeded in developing a faster process in the laboratory that reduces the amount of residues and reagents by more than 90%,” Araki said.
Applications against COVID-19
The initial idea regarding applications was to use the molecule to eliminate unpleasant odors produced by microorganisms in textiles and to remove germs in various kinds of environment. With the advent of COVID-19, the researchers took the initiative of finding out whether the compound could cause oxidative damage to SARS-CoV-2 and inactivate it. To this end, they contacted Edison Luiz Durigon, a professor at ICB-USP who heads a Biosafety Level 3 (BS-3) laboratory designed to handle pathogens that can cause serious and potentially lethal diseases, including SARS-CoV-2.
Durigon and his group were the first in Brazil to isolate the novel coronavirus and grow it in the laboratory, using samples donated by the first patients diagnosed with the disease at the Albert Einstein Jewish Hospital (HIAE) in São Paulo (more at: agencia.fapesp.br/32699/).
They were then contacted by startups and other entities interested in testing the efficacy of diagnostic tests and products developed to combat COVID-19, such as silver-silica nanoparticles with virucidal action applied to the surface of various kinds of material. The nanoparticles are produced by Nanox, a startup supported by the FAPESP Innovative Research in Small Business Program (PIPE).
“Our lab has tested several antivirals that work against SARS-CoV-2, but none at such low concentrations as this molecule,” Durigon said. “It displays immediate action against coronavirus. The oxidation reactions it triggers destroy the virus’s lipoprotein envelope.”
Once the molecule’s action had been proven, Golden Technology developed an antiviral surgical mask coated with the substance and launched the product in July 2020.
According to the researchers, the material’s antiviral effect and bacterial filter efficiency (BFE) last 12 hours. The mask can be worn for three hours per day for four days, for example, before it becomes ineffective.
“In the mask and fabric, the molecule’s antiviral action lasts a long time. Our trials showed that the compound remains active in these materials for up to 12 hours of use,” Durigon said.
Oral hygiene products
Before the pandemic, Golden Technology’s researchers had also started working with TRIALS Saúde Bucal e Tecnologias, a firm based in Bauru, on a line of oral hygiene products containing the molecule, such as toothpaste, mouthwash, spray and dental gel.
“The idea was to use the oxidizing chemical reactions promoted by the molecule to help repair soft tissue in the mouth, treat gingivitis, and eliminate halitosis,” said Fabiano Vieira Vilhena, who owns TRIALS.
The pandemic changed the focus for the clinical trials. The tests performed on cultured cells by Durigon’s group showed that the molecule could inactivate SARS-CoV-2, so the researchers decided to see if the mouthwash containing the compound could reduce viral load in the saliva of infected patients.
One of the first in vitro studies, conducted by researchers at FOB-USP in partnership with colleagues at São Paulo State University (UNESP) in Botucatu, showed that an antiseptic mouthwash with phthalocyanine reduced viral load in saliva samples by 90%. A paper on the results was published in Clinical, Cosmetic and Investigational Dentistry.
“Thanks to this study, we proved the molecule to be able to inactivate the virus in saliva,” said Paulo Sérgio da Silva Santos, a professor at FOB-USP and principal investigator for the clinical trials.
Based on reports in the literature that infection by SARS-CoV-2 starts with viral entry and replication in oral and nasal mucosa, the researchers decided to find out whether oral rinsing and gargling with an antiseptic mouthwash containing the compound could reduce viral load in the mouth and throat in the initial stage of infection and improve the clinical response in COVID-19 patients.
“Contact with and colonization of oral and nasal mucosa by SARS-CoV-2 happens in the initial stage of the disease. Viral load in the mouth diminishes a great deal after seven days. The window of opportunity for the mouthwash to be effective is therefore the onset of infection,” Santos said.
To confirm this hypothesis, a clinical trial was performed involving 41 patients diagnosed with COVID-19 by RT-PCR. They had mild or moderate symptoms and were undergoing treatment at Bauru State Hospital in May 2020.
The results showed that the average hospital stay was significantly shorter for a group of 20 patients treated with the antiseptic mouthwash containing the molecule five times a day for one minute until discharge than for a group treated with a mouthwash that did not contain the molecule.
Patients who used the mouthwash with the molecule stayed in hospital for four days on average, compared with seven for those who used the mouthwash without the molecule. The treatment also reduced symptom severity.
None of the patients who used the mouthwash with the molecule required intensive care or died. They were 15 years older on average than the patients who used the mouthwash without the molecule.
“All patients were given the same standard hospital care for COVID-19 recommended by the WHO [World Health Organization], save only that the group of 20 patients used the mouthwash with the molecule,” Santos said. “We were able to show statistically that the only factor that influenced the shorter hospital stay was use of the mouthwash containing the molecule.”
Adjuvant therapy
The researchers plan to conduct more studies in order to see how long the mouthwash with the molecule continues to act on oral mucosa. The ability of an antimicrobial agent to retain its effectiveness in the mouth for an extended period is known in medicine as substantivity.
The compounds used in conventional disinfectants, such as chlorhexidine (which does not inactivate SARS-CoV-2), remain active in the mouth for eight to 12 hours. In the case of iron phthalocyanine, preliminary results suggest it adheres to oral mucosa and has a virucidal effect for up to two hours, with residual action in the oropharyngeal cavity.
“Because the molecule’s effect resembles that of oxygenated water but is produced locally in small amounts the whole time, its toxicity is insignificant,” Araki said.
The company that will commercialize the mouthwash and other oral hygiene products containing the molecule is adjusting the formulas to obtain registration with ANVISA, the national health surveillance agency and the body responsible for regulating medical drugs in Brazil.
If the products are approved, they can be used in adjuvant therapy, reducing viral load in the initial stage of infection while the immune system is preparing to produce antibodies and combat the virus.
“Reducing viral load slows down the infection and gives the immune system time to combat it. By the time viral load becomes higher in other tissues not reached by the mouthwash, the immune system will be sufficiently active,” Durigon said.
The Clinical, Cosmetic and Investigational Dentistry article “Virucidal activity of the antiseptic mouthwash and dental gel containing anionic phthalocyanine derivative: in vitro study” is at: www.dovepress.com/virucidal-activity-of-the-antiseptic-mouthwash-and-dental-gel-containi-peer-reviewed-fulltext-article-CCIDE.
The Scientific Reports article “Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomized trial” is at: www.nature.com/articles/s41598-021-99013-5.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





