

Molecule DF2593A is being developed by the Center for Research on Inflammatory Diseases in partnership with the Italian pharmaceutical company Dompé
Molecule DF2593A is being developed by the Center for Research on Inflammatory Diseases in partnership with the Italian pharmaceutical company Dompé
Molecule DF2593A is being developed by the Center for Research on Inflammatory Diseases in partnership with the Italian pharmaceutical company Dompé
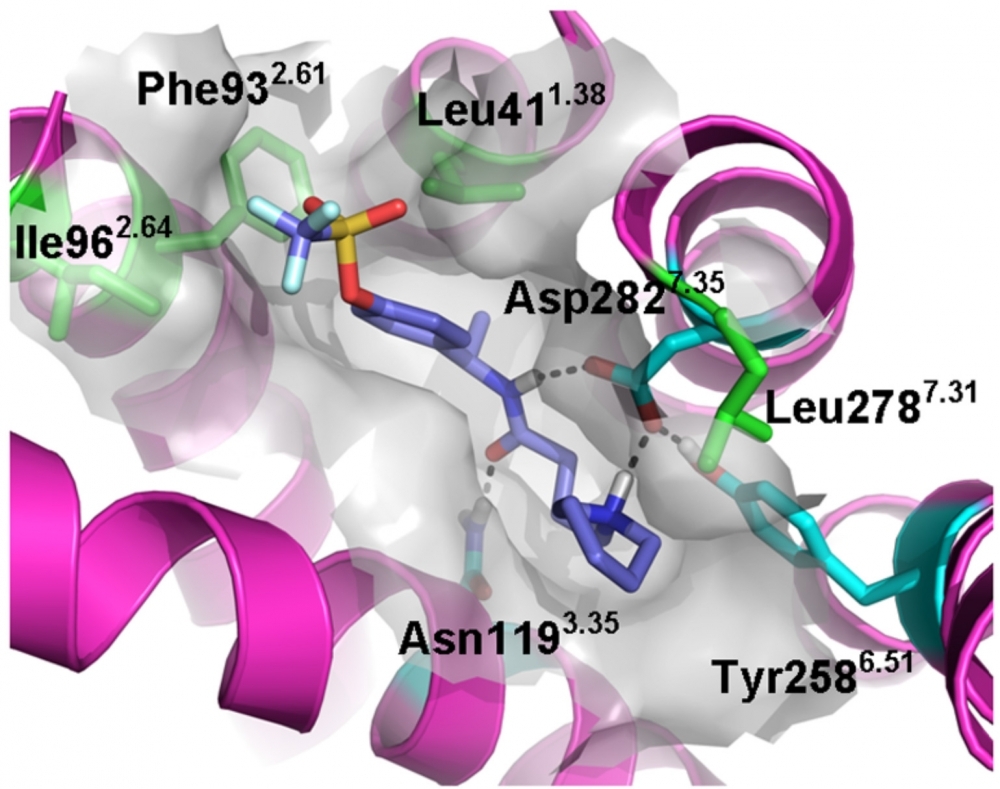
Molecule DF2593A is being developed by the Center for Research on Inflammatory Diseases in partnership with the Italian pharmaceutical company Dompé
By Karina Toledo
Agência FAPESP – A new molecule known as DF2593A has been shown in animal testing to relieve up to 80% of the pain resulting from acute and chronic inflammatory conditions. In models of neuropathic pain caused by injury to nerves, pain reduction reached 60%.
The Center for Research on Inflammatory Diseases (CRID), one of the Research, Innovation & Diffusion Centers (CEPIDs) supported by FAPESP, is conducting the research project in partnership with Dompé, an Italian pharmaceutical company.
Some of the results of preclinical tests were described in November in the journal Proceedings of the National Academy of Sciences (PNAS).
“At the moment we’re performing toxicological tests, and if they’re successful we can then plan clinical trials. Our aim is to develop a new drug for pain relief with fewer adverse effects than the anti-inflammatory drugs currently available,” said Thiago Mattar Cunha, a professor at the University of São Paulo’s Ribeirão Preto School of Medicine (FMRP-USP) and one of the authors of the article.
The target for the new drug is a peptide known as C5a, a component of the so-called complement system that plays a mediating role in inflammatory pain, as reported by the CRID group in an article published in 2007 by the British Journal of Pharmacology.
“The complement system is a set of plasma proteins that are activated in a series of cascading reactions,” Cunha explained. “In other words, each activated component is capable of activating another component of the system. This occurs during inflammatory or infectious processes.”
“Among the components of this system, C5a is one of the peptides produced systemically or at the site of the inflammation when the complement system is activated,” he said. “When C5a is produced, it has various biological effects, both local and systemic. It is a proinflammatory molecule, playing a role in leukocyte activation and in the production of free radicals and inflammatory cytokines.”
To produce its biological effects, Cunha added, the peptide must bind to a receptor called C5aR, which belongs to the large family of G protein-coupled receptors (which are important for cell signaling) and is expressed by many cell types. “So we had the idea of developing a molecule that could relieve pain by blocking this receptor.”
Alternative binding
In partnership with researchers at Dompé, the CRID group had been investigating molecules capable of blocking the receptors of chemokines, a type of inflammatory cytokine, in an allosteric manner.
“An allosteric blocker is a molecule capable of binding to a receptor at a site distinct from that of the orthosteric agonist [physiological ligand],” Cunha explained. “That is, the agonist still binds to the receptor, but the receptor isn’t activated. This is a highly selective kind of modulation.” The researchers discovered that C5aR also presented a possible allosteric site, indicating that it might be possible to identify substances capable of selectively modulating the action of this receptor.
Experts in medicinal chemistry at Dompé then pursued ways to modify the structure of chemokine receptor inhibitors and to synthesize new compounds capable of binding allosterically to C5aR.
“The prototype called DF2593A was the one that displayed the most powerful action on C5aR without interfering with the chemokine receptor,” Cunha said. Next, a series of in vitro and in vivo tests was carried out to prove the new inhibitor’s action in human, mouse and rat cells, and the researchers calculated the dose necessary to obtain therapeutic effects in each organism.
Cunha described the work in detail. “We performed trials to show that the action is highly specific to the complement system and doesn’t interfere with receptors of the adrenergic systems, such as cannabinoids, among others. In one of the tests we altered the amino acids at the allosteric site of C5aR and found that when this happens the compound ceases to be active. This proves our hypothesis that DF2593A binds in a highly selective manner to the allosteric site of C5aR.” The researchers also performed pharmacokinetic tests, which showed that the compound is absorbed orally and remains in the blood plasma for at least 12 hours after administration.
The next step was oral DF2593A treatment of several animal models of chronic and acute inflammation, as well as models of neuropathic pain resulting from traumatic sciatic nerve injury. Neuropathic pain may also be caused by diabetes and other diseases or by the adverse effects of chemotherapy drugs. Chronic inflammatory pain is common in conditions such as rheumatoid arthritis and arthrosis.
According to Cunha, “The most interesting finding is that the compound blocks inflammatory pain without affecting physiological pain, which is important for survival. That is, the capacity to respond to painful stimuli is maintained in uninflamed animals when treated with DF2593A, which is not the case when morphine derivatives are administered, for example.”
Moreover, he went on, the compound did not have a sedative effect on rodents. If the new analgesic is successful in clinical trials, Cunha believes that it may become a good therapeutic alternative to the anti-inflammatory drugs available today.
“The drugs currently used in clinical treatment inhibit cyclooxygenase (COX) enzymes, which, besides playing a role in inflammation, are important for homeostasis of the gastric, cardiac and renal systems, hence their significant side effects in the long run. In contrast, DF2593A acts only on C5aR, which doesn’t play a major physiological role. We studied a transgenic mouse model that doesn’t express C5aR, and the animal is completely normal. It even displays greater resistance to infection,” Cunha concluded.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





