

Researchers describe how fumiquinazoline C is synthesized and investigate its function in conidia, asexual non-motile spores of the fungus Aspergillus fumigatus that can cause severe infections in immunosuppressed patients. The discovery could lead to the development of novel medications (image: Aspergillus fumigatus conidia/CDC)
Researchers describe how fumiquinazoline C is synthesized and investigate its function in conidia, asexual non-motile spores of the fungus Aspergillus fumigatus that can cause severe infections in immunosuppressed patients. The discovery could lead to the development of novel medications.
Researchers describe how fumiquinazoline C is synthesized and investigate its function in conidia, asexual non-motile spores of the fungus Aspergillus fumigatus that can cause severe infections in immunosuppressed patients. The discovery could lead to the development of novel medications.
Researchers describe how fumiquinazoline C is synthesized and investigate its function in conidia, asexual non-motile spores of the fungus Aspergillus fumigatus that can cause severe infections in immunosuppressed patients. The discovery could lead to the development of novel medications (image: Aspergillus fumigatus conidia/CDC)
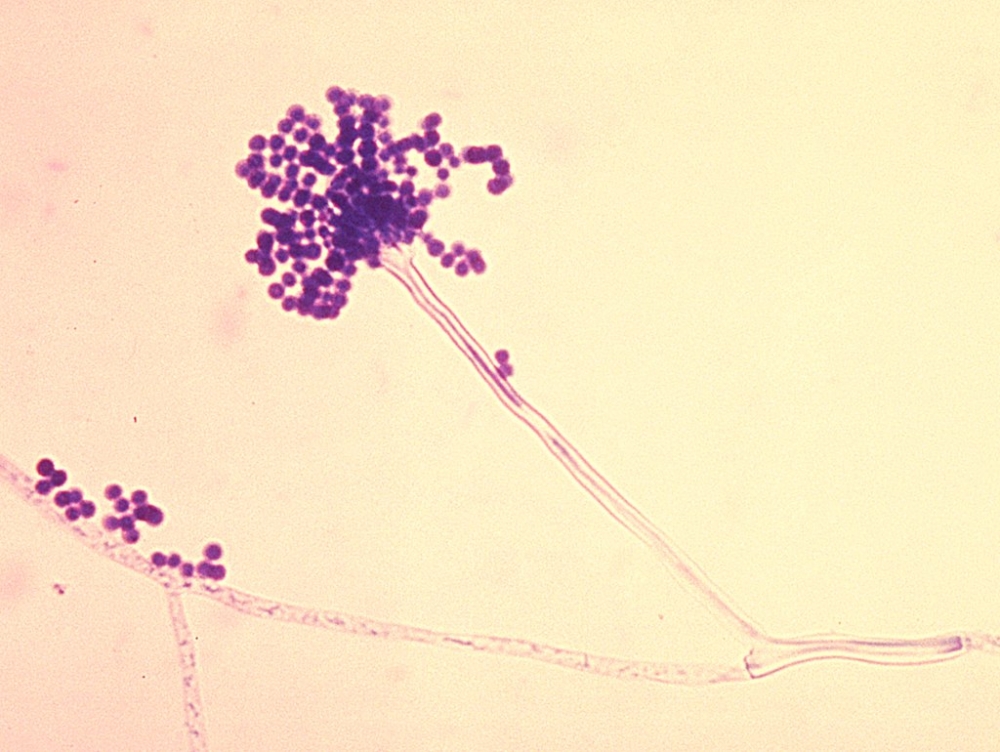
By André Julião | Agência FAPESP – A group of researchers supported by FAPESP has described how the fungus Aspergillus fumigatus regulates the production of fumiquinazoline C and probably performs a protective function against other microorganisms and defense cells in the mammalian immune system. A. fumigatus, a spore-forming mold, is responsible for some 90% of cases of invasive pulmonary aspergillosis, a disease that affects patients with a compromised immune system and kills between 60% and 90%. The discovery may lead to the development of novel drugs.
The researchers report their findings in an article published in the journal Genetics.
“Fumiquinazoline C is a secondary metabolite produced by A. fumigatus, meaning the metabolic pathways of its biosynthesis aren’t directly associated with the production of chemical energy in the cell. Its production actually requires a lot of energy, which comes from the organism’s base metabolism, so its function should necessarily permit an adaptation or a gain for the organism to persist in the environment,” said Iran Malavazi, principal investigator for the study. Malavazi is a professor in the Department of Genetics and Evolution at the Federal University of São Carlos’s Center for Biological Sciences and Health (CCBS-UFSCar) in the state of São Paulo, Brazil.
The substance is one of the few secondary metabolites produced in cells. It accumulates in the conidia, asexual non-motile spores of the fungus. A. fumigatus lives in soil, thrives in practically all environments worldwide, and is capable of adapting to various types of stress. Its conidia are so abundant in the air that we inhale 100-200 per day, according to scientific estimates.
“These structures normally enter the organism via the airways and are phagocytized by our innate defense cells, which prevent infection,” Malavazi said. “The process is different in immunosuppressed patients, however. Patients admitted to intensive care units or receiving treatment that inhibits the immune system, such as transplant patients or people with cancer, are extremely vulnerable to this type of infection. Most of those infected die.”
The study was part of the PhD research of first author Marina Campos Rocha at CCBS-UFSCar, and part of a project funded by FAPESP.
Phagocytosis inhibitor
In a series of experiments, the researchers discovered that the functions of fumiquinazoline C probably include protecting conidia by poisoning cells that perform phagocytosis such as defense cells or competing microorganisms in the soil such as bacteria and protozoans. In phagocytosis, the cells use their own structure to engulf the invader and prevent it from acting. Fumiquinazoline C inhibits this process.
To arrive at this result, the researchers produced mutant versions of A. fumigatus by editing its DNA so that it produced, on one hand, conidia with up to 4.5 times more fumiquinazoline C, and conidia with 50%-95% less than in the wild type on the other. In one experiment the conidia were placed with free-living amoebae of the slime mold species Dictyostelium discoideum, which inhabit soil with A. fumigatus and compete for the same resources.
In the mutants that produced more fumiquinazoline C, the amoebae phagocytized fewer conidia, and vice-versa (mutants that produced less were more phagocytized). To confirm the role of the substance in this competition, the researchers isolated it on a large scale for the first time and applied it in a medium containing amoebae and conidia that produced little fumiquinazoline C. The result was that the conidia were once again less phagocytized.
Another experiment replaced the amoebae with macrophages, a type of defense cell, isolated from mice. The results were similar, suggesting a probable role for fumiquinazoline C in infections in humans and an additional mechanism whereby conidia from this fungus interact with the innate immune system.
The group was able to conclude that the substance performs this important function in the fungal life cycle thanks to its discovery of a cell signaling cascade that has been the main focus of the research conducted by Malavazi. They were interested above all in what is known as the cell wall integrity pathway, which synthesizes, repairs and remodels cell walls, and is, therefore, a potential target for novel medications. The results of the study showed for the first time the involvement of this signaling pathway in the production of a secondary metabolite in A. fumigatus.
The researchers now have data that will soon enable them to deepen their understanding of the role played by fumiquinazoline C when it comes into contact with human cells. They also plan to investigate whether the fungus produces an antidote to the substance so that it is not killed by its own poison, an outcome already observed in both A. fumigatus and other microorganisms that produce toxins (more at agencia.fapesp.br/34133). Their findings could pave the way for the development of more effective drugs against invasive aspergillosis.
The co-authors of the article include Gustavo Henrique Goldman, a professor at the University of São Paulo’s Ribeirão Preto School of Pharmaceutical Sciences (FCFRP-USP) and also supported by FAPESP.
In addition to the teams affiliated with UFSCar and USP, researchers at São Paulo State University (UNESP), the University of Campinas (UNICAMP) and the Federal University of the ABC (UFABC) in Brazil also participated in the study, as well as a scientist working at Hans Knöll Institute (HKI) in Germany.
The article “Transcriptional control of the production of Aspergillus fumigatus conidia-borne secondary metabolite fumiquinazoline C important for phagocytosis protection” can be retrieved from: academic.oup.com/genetics/advance-article-abstract/doi/10.1093/genetics/iyab036/6168429.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





