

The researchers at USP analyzed lung tissue from nine patients who died from COVID-19 (illustration: Elia Caldini)
In an article published in the Journal of Applied Physiology, researchers at the University of São Paulo show that damage to small blood vessels in the lungs caused by SARS-CoV-2 is the main factor underlying severe COVID-19.
In an article published in the Journal of Applied Physiology, researchers at the University of São Paulo show that damage to small blood vessels in the lungs caused by SARS-CoV-2 is the main factor underlying severe COVID-19.
The researchers at USP analyzed lung tissue from nine patients who died from COVID-19 (illustration: Elia Caldini)
By Maria Fernanda Ziegler | Agência FAPESP – Blood clotting (thrombosis) in the capillary vessels of the lungs is one of the first consequences of severe COVID-19, even preceding the respiratory distress caused by diffuse alveolar damage, according to a Brazilian study reported in an article published in the Journal of Applied Physiology. Autopsies of nine patients who died after developing the severe form of the disease showed a clearly typified condition involving alterations to lung vascularization and thrombosis.
For the first time, the article describes sub-cellular aspects of the endothelial damage and associated thrombotic phenomena caused by the infection. It notes the impact of acute inflammation on lung microvascular circulation as the key factor in severe COVID-19, contributing to a deeper understanding of the pathophysiology of the disease and the development of novel therapeutic strategies.
“This study furnished the final proof of what we’d been pointing out since the very start of the pandemic – that severe COVID-19 is a thrombotic disease. The virus SARS-CoV-2 has tropism for [is attracted to] the endothelium, the layer of cells that lines blood vessels. When it invades endothelial cells, it first affects microvascular circulation. The problem starts in the capillaries of the lungs [the tiny blood vessels that surround the alveoli], followed by clotting in the larger vessels that can reach any other organ,” said pulmonologist Elnara Negri, first author of the article and a professor at the University of São Paulo’s Medical School (FM-USP). She was one of the first researchers in the world to reach the conclusion that severe COVID-19 is a thrombotic disease (read more at: agencia.fapesp.br/33233).
In the study, which was supported by FAPESP, the researchers used transmission and scanning electron microscopy to observe the effects of the virus on lung endothelial cells from severe COVID-19 patients who died at Hospital das Clínicas, the hospital complex operated by FM-USP.
All nine samples obtained by minimally invasive autopsies displayed a high prevalence of thrombotic microangiopathy – microscopic blood clots in small arteries and capillaries that can lead to organ damage and ischemic tissue injury. The samples came from patients who were hospitalized between March and May 2020, required intubation and intensive care, and died owing to refractory hypoxemia and acute respiratory failure.
It is worth noting that none of the patients included in the study was treated with anti-coagulants, as this was not part of the COVID-19 treatment protocol at the time. Nor were any COVID-19 vaccines available in the period.
Endothelial glycocalyx shedding
Negri explained that the endothelium is itself lined by a gel-like layer of glycoproteins called the glycocalyx, which acts as a barrier to regulate the access of macromolecules and blood cells to the endothelial surface. This barrier prevents clotting in blood vessels by inhibiting platelet interaction with the endothelium.
“Previous studies conducted by Helena Nader at UNIFESP [the Federal University of São Paulo] showed that SARS-CoV-2 invades cells mainly by binding to the receptor ACE-2 [a protein on the surface of various cell types, including epithelial and endothelial cells in the respiratory system] but before that, it binds to heparan sulphate [a polysaccharide], a major component of the glycocalyx in endothelial cells. When it invades the endothelium, it triggers shedding and destruction of the glycocalyx, resulting in tissue exposure and intravascular clotting. The process starts in the microcirculation,” Negri explained.
Because the virus initially acts on the pulmonary microcirculation, contrast examinations performed during the pandemic to investigate the presence of blood clots in larger vessels in severe COVID-19 patients failed to detect the problem at any early stage, she added. However, endothelial dysfunction is a key phenomenon in COVID-19 since it is directly associated with activation of the inflammatory response that is characteristic of the disease.
“Massive viral invasion and destruction of the endothelium break down the endothelial barrier and impair the recruitment of circulating immune cells, activating pathways associated with thrombogenesis and inflammation,” she said.
In the study, the researchers found that endothelial injury tended to precede two common processes in cases of respiratory distress: significant alveolar capillary membrane leakage, and intra-alveolar accumulation of fibrin (associated with blood clotting and wound healing).
A study by the same group at FM-USP, led by Thais Mauad and including transcriptomics (analysis of all RNA transcripts, coding and non-coding), showed that several pathways associated with blood clotting and platelet activation had been activated prior to inflammation in the lungs of patients with alveolar damage.
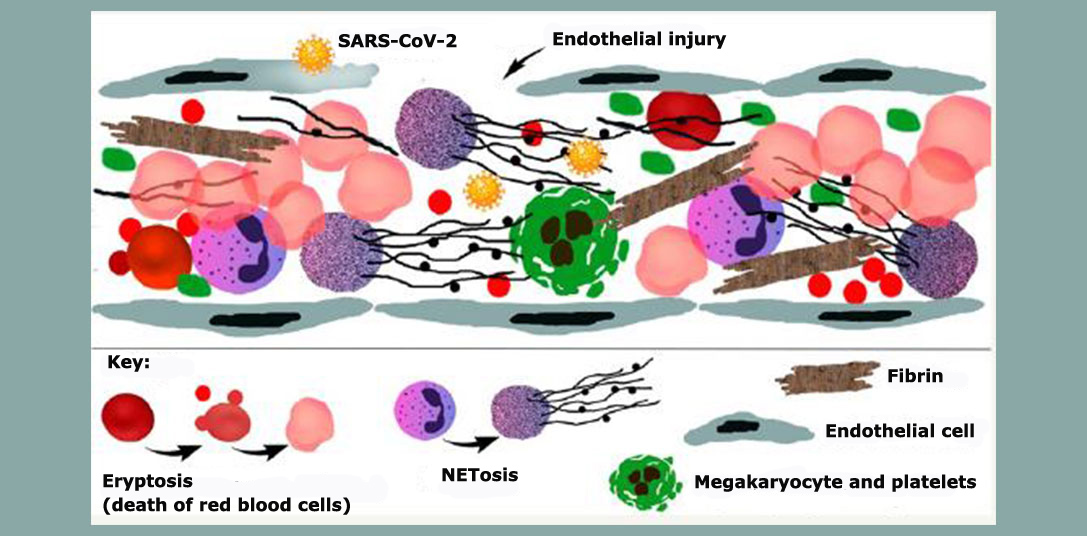
The analysis also confirmed that the clotting was not typical of the usual process triggered by activation of coagulation factors. “In COVID-19, the clotting is due to endothelial injury and exacerbated by NETosis [an immune mechanism involving programmed cell death via formation of neutrophil extracellular traps or NETs], dysmorphic red blood cells and platelet activation, all of which makes the blood thicker and causes many complications,” Negri said.
When the blood is thick and highly thrombogenic, she added, the patient must be kept hydrated, whereas diffuse alveolar damage in acute respiratory distress syndromes due to other causes requires reduced hydration. “Also, the timing and rigorous control of anti-coagulation are fundamental,” she stressed.
Another study by the same group of researchers, including Marisa Dolhnikoff and Elia Caldini, showed lung damage in severe COVID-19 to be associated with the degree of NETosis: the higher the level of NETs in lung tissue obtained by autopsy, the more the lungs were damaged.
Negri said she began to suspect there was a link between COVID-19 and thrombosis early in the pandemic when she noticed a phenomenon recalling her experience some 30 years ago with patients who had microvascular clotting after open-heart surgery with extracorporeal circulation and a bubble oxygenator, no longer used because it causes endothelial damage.
“It was a widely used technique 30 years ago, but it causes lung injury very similar to that seen in COVID-19. So I’d already seen it. Besides the pulmonary injury, another similarity is the occurrence of peripheral thrombotic phenomena, such as red toes, for example,” she said.
“As severe COVID-19 sets in, the drop in blood oxygen levels is secondary to pulmonary capillary thrombosis. Initially, there’s no buildup of fluid in the lungs, which aren’t ‘saturated’ and don’t lose their compliance or elasticity. This means the lungs in early severe COVID-19 patients don’t look like sponges full of liquid, as they do in acute respiratory distress syndrome [ARDS] patients. On the contrary, the respiratory failure associated with severe COVID-19 involves dehydration of the lungs. The alveoli fill with air but the oxygen can’t enter the bloodstream because of capillary clotting. This leads to what we call ‘happy hypoxia’, where patients don’t experience shortness of breath and aren’t aware their oxygen saturation is dangerously low.”
While observing the intubation of a severe COVID-19 patient, Negri realized the treatment of such cases should be entirely different from what it was at the start of the pandemic. “The secret to treating severe COVID-19 patients is keeping them hydrated and using anti-coagulant at the right dose, meaning the dose required in the hospital environment at the onset of oxygen desaturation, i.e. low levels of oxygen in the blood,” she said. “After that, the therapeutic dose of anti-coagulant must be calculated daily on the basis of blood work, always in the hospital environment to avoid any risk of bleeding. Prophylaxis is required for an average of four to six weeks after discharge because that’s how long the endothelium takes to regenerate.”
This hydration and anti-coagulation protocol is needed because, in contrast with other kinds of ARDS in which oxygen in the lungs is prevented from entering the bloodstream mainly by alveolar inflammation, lung capillary endothelial damage is the main obstacle in early severe COVID-19, she explained.
“No one knew about this difference between COVID-19 and other types of ARDS at the very start of the pandemic. Indeed, this is why so many Italian patients died in ICUs [intensive care units], for example. The treatment protocol used then was different,” she recalled.
In 2020, before the study reported in the Journal of Applied Physiology, Negri and her group had already observed that use of the anti-coagulant heparin improved oxygen saturation in critical patients. In 2021, in collaboration with colleagues in several countries, they conducted a randomized clinical trial in which they succeeded in demonstrating that treatment with heparin reduced severe COVID-19 mortality. The findings were published in the British Medical Journal (read more at: agencia.fapesp.br/37148).
“That study helped bring about a global change in COVID-19 treatment guidelines by showing that COVID-19 mortality risk fell 78% when anti-coagulation was started in patients who needed oxygen supplementation but weren’t yet in intensive care,” Negri said.
Endothelial dysfunction should be reversed without delay in severe COVID-19, using anti-coagulant, she explained. “Blood clotting has to be stopped as soon as possible in order to avert the development of acute respiratory distress and other consequences of the disease, such as the problems now known as long COVID,” she said.
An article recently published in Nature Medicine by researchers affiliated with institutions in the United Kingdom reinforces the thrombotic nature of the disease, reporting a study in which the only long COVID prognostic markers identified were fibrinogen and D-dimer, proteins associated with coagulation.
“The study shows that long COVID results from inadequately treated thrombosis. The microcirculatory problem can persist in several organs, including the brain, heart and muscles, as if the patient were having small heart attacks,” Negri said.
The article “Ultrastructural characterization of alveolar microvascular damage in severe COVID-19 respiratory failure” is at: journals.physiology.org/doi/abs/10.1152/japplphysiol.00424.2023.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.





